About Production
Global Nutrition SolutionsStart Right Here.
GC Wellbeing’s Innovative Future
With the establishment of Eumseong Plant, a manufacturing site specialized in existing placenta preparations, Seongnam Plant, a manufacturing site for health supplements & foods, and the new Innovation Plant, GC Wellbeing is Solidifying its position as the supplier of safer and more effective pharmaceuticals and health supplements & foods.
Innovation Plant
- Land Area 33,057㎡
- Floor Area 18,512㎡
- Annual production capacity of 27 million vials and 36 million ampules
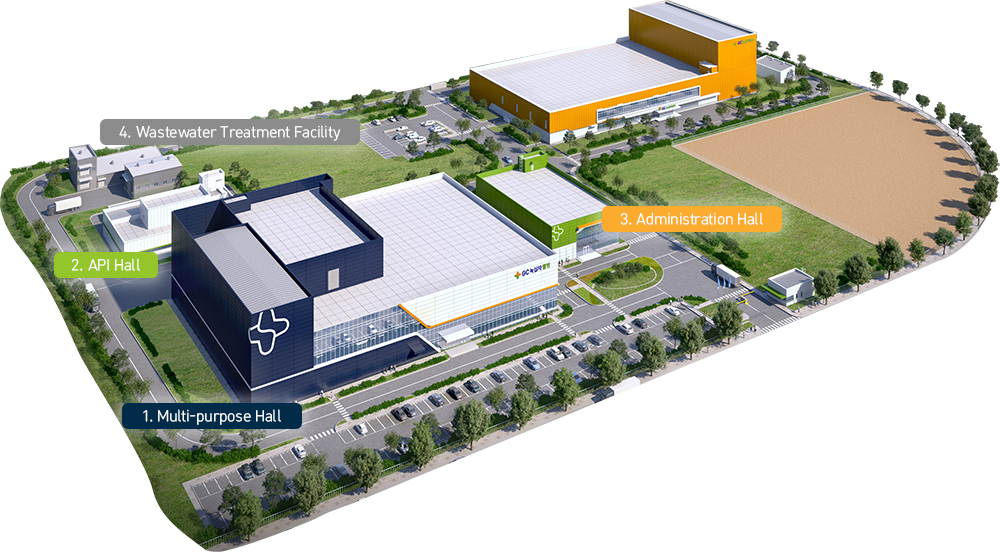

1. Multi-purpose HallFunction: Production, utilities, logistics & raw ingredient management, quality control, and quality assurance
- Production, logistics utilities, and quality management
- Includes refining room with Korea’s only placenta hydrolysis treatment equipment
- State-of-the-art GMP facilities required for production
- KGCSP warehouse—a pharmaceutical logistics space able to store 2,000 cells at room temperature and a height of 25m above the floor
2. API HallFunction: Production of placental drugs and substances
- A place for recycling of placenta (classified as medical waste) to produce the raw ingredients needed
- Korea’s largest placental treatment facility with the best explosion-proof equipment for placental degreasing using acetone
3. Administration HallFunction: Support operation of Innovation Plant
- Support office, conference rooms (large and small), auditorium, reception room, library, infirmary, cafeteria, a lounge for female employees, café, etc.
- Supporting Innovation Plant employees with five work support areas and five welfare areas
4. Wastewater Treatment FacilityFunction: Waste and hazardous material storage (from Innovation Plant)
- Waste management facility with 200 tons per day treatment capacity.
- Treats wastewater from GC Wellbeing and GCMS
Eumseong Plant
- A manufacturing site specialized in existing placenta preparations
- A thorough virus test, Placenta history management, Clean collection and manufacturing system.
- Report and approve DMF
- Production of Laennec, a plancetal injection, and Fursultamine, GCVITAFIVE
Seongnam Plant
- Manufacturing health supplements & foods
- Research and development of individually-approved materials
- KFDA’s FGMP certification acquired